Revita Life Sciences
Knowledge
Stem Cell
Stem cells are biological building blocks, the starting point of human life. However, with proper direction, they are more useful when treating disease. It is a cell that does not have a definite role yet. It can regenerate itself and is capable of self-renewing. This cell remains quiescent until activated. This kind of cell is usually found among different cells of an organ.
A deeper understanding of stem cell instruction could yield a deeper understanding of the origins of specific diseases.
Only non-ESC have been used clinically so far. Bone marrow cells were first used successfully 4 decades ago, and cord blood stem cells in the past 10–15 years. These cells have been beneficial for blood disorders such as leukemia, multiple myeloma and lymphoma; and disorders with defective genes such as severe combined immune deficiency.
While the potential of stem-cell therapy to revolutionize medicine is undeniable, it’s important to note that the majority of treatments being developed are still in the experimental stage or under clinical trial. The exception to this is bone marrow derived Mesenchymal Stem Cells transplants, which have shown promising results.
Unrivalled

Bone Marrow derived Mesenchymal Stem Cells
One of the most promising cellular sources is bone-marrow-derived mesenchymal stem cells (MSCs) also termed multipotent stromal cells. MSCs represent an autologous source and are not only abundant but also non-tumorigenic, ensuring their safety and availability for therapeutic applications. Additionally, MSCs possess the useful characteristics of homing and chemokine secretion.
The mechanisms by which transplanted MSCs influence diseases can be broadly classified as cellular replacement or paracrine secretion, with the latter subdivided into trophic factor secretion or immunomodulation by cytokines. Emerging research suggests that genetic manipulations before transplantation could enhance the therapeutic potential of MSCs. Such manipulation could turn the cells into a ‘Trojan horse’ to deliver specific proteins or promote reprogramming of the MSCs into the neural lineage.

Adipose tissue derived stem cells
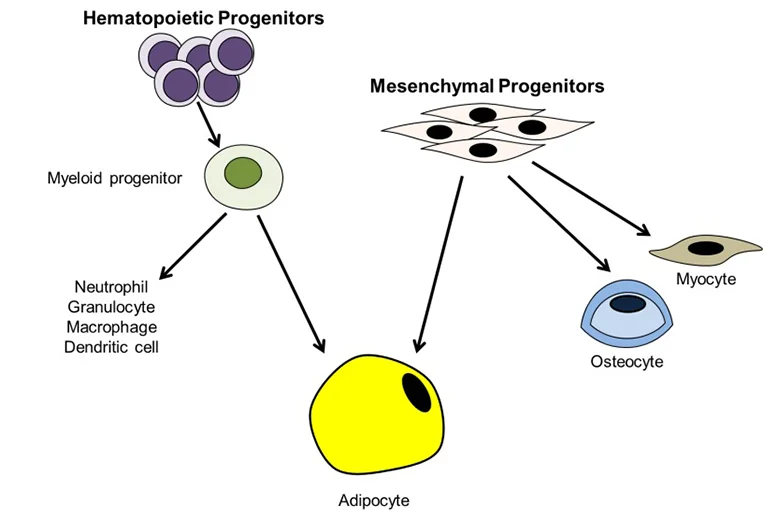
Adipose tissue-derived stem cells (ADSC) are routinely isolated from homogenized adipose tissues stromal vascular fraction (SVF). Like other types of mesenchymal stem cells (MSC), ADSC remains challenging to define due to the lack of definitive cellular markers. Still, many types of MSC, including ADSC, have been shown to reside in a perivascular location, and increasing evidence shows that both MSC and ADSC may be vascular stem cells (VSC). Locally, these cells differentiate into smooth muscle and endothelial cells assembled into newly formed blood vessels during angiogenesis and neovasculogenesis.
MSC or ADSC can also differentiate into tissue cells such as adipocytes in the adipose tissue. Systematically, MSCs or ADSCs are recruited to injury sites where they participate in the repair/regeneration of the injured tissue. Due to the vasculature’s dynamic capacity for growth and multipotential nature for diversification, VSC in tissue are individually at various stages and on different paths of differentiation. Therefore, when isolated and put in culture, these cells are expected to be heterogeneous in marker expression, renewal capacity, and differentiation potential. Although this heterogeneity of VSC does impose difficulties and cause confusions in basic science studies, its impact on the development of VSC as a therapeutic cell source has not been as apparent, as many preclinical and clinical trials have reported favorable outcomes. With this understanding, ADSC are generally defined as CD34+CD31- although loss of CD34 expression in culture is well documented. In adipose tissue, CD34 is localized to the intima and adventitia of blood vessels but not the media where cells expressing alpha-smooth muscle actin (SMA) exist.
By excluding the intima, which contains the CD34+CD31+ endothelial cells, and the media, which contains the CD34-CD31- smooth muscle cells, it leaves the adventitia as the only possible location for the CD34+ ADSC. In the capillary, CD34 and CD140b (a pericyte marker) are mutually exclusively expressed, thus suggesting that pericytes are not the CD34+ ADSC. Many other cellular markers for vascular cells, stem cells, and stem cell niche have also been investigated as possible ADSC markers. Particularly the best-known MSC marker STRO-1 has been found either expressed or not expressed in cultured ADSC. In the adipose tissue, STRO-1 appears to be expressed exclusively in the endothelium of certain but not all blood vessels, and thus not associated with the CD34+ ADSC. In conclusion, we believe that ADSC exist as CD34+CD31-CD104b-SMA- cells in the capillary and in the adventitia of larger vessels. In the capillary these cells coexist with pericytes and endothelial cells, both of which are possibly progenies of ADSC (or more precisely VSC). In the larger vessels, these ADSC or VSC exist as specialized fibroblasts (having stem cell properties) in the adventitia.
Blood Stem Cells
A small number of hematopoietic stem cells circulate in the bloodstream. These cells, with the same properties as bone marrow stem cells , can be used as a substitute. The circulating blood stem cells , obtained from drawn blood, offer a less-invasive procedure than collecting bone marrow cells , providing a more comfortable and reassuring experience for both patients and medical professionals. However, due to their scarcity, collecting enough of them can be challenging.
The mechanisms by which transplanted MSCs influence diseases can be broadly classified as cellular replacement or paracrine secretion, with the latter subdivided into trophic factor secretion or immunomodulation by cytokines. Emerging research suggests that genetic manipulations before transplantation could enhance the therapeutic potential of MSCs. Such manipulation could turn the cells into a ‘Trojan horse’, to deliver specific proteins, or promote reprogramming of the MSCs into the neural lineage.
Umbilical Cord Blood Stem Cells

After a baby is born, the umbilical cord can be set aside and used as a source of stem cells. The umbilical cord blood is a rich source of stem cells that can be substituted for bone marrow stem cells.
Umbilical cord blood stem cells are rejected less often by the host tissue, possibly because they have not developed cell-surface molecules that can be recognized and attacked by the host’s immune system.
The mechanisms by which transplanted MSCs influence diseases can be broadly classified as cellular replacement or paracrine secretion, with the latter subdivided into trophic factor secretion or immunomodulation by cytokines. Emerging research suggests that genetic manipulations before transplantation could enhance the therapeutic potential of MSCs. Such manipulation could turn the cells into a ‘Trojan horse’, to deliver specific proteins, or promote reprogramming of the MSCs into the neural lineage.
Umbilical Cord tissue Stem Cells

Mesenchymal stem cells (MSCs) can be isolated from many tissues, such as bone marrow, adipose tissue, umbilical cord etc. However, umbilical cord tissue, especially the part known as “Wharton’s Jelly”, is also a source of mesenchymal stem cells. The umbilical cord is routinely discarded at parturition, thus the isolation of MSCs from this tissue raises no ethical controversy compared with the ethical concerns associated with embryonic stem cells. MSCs from this tissue have greater differentiation potential and faster rates of division compared to bone marrow-derived MSCs. The large volume of umbilical cord and ease of physical manipulation increases the yield of MSCs. Also, the capacity of MSCs cells to proliferate is known to decrease with the age of the donor. Due to the presence of placental barrier, umbilical cord MSCs have lower risk of bacterial and viral infections than those isolated from bone marrow, adipose tissue and peripheral blood.
The efficiency of processing and isolation of mesenchymal stem cells from umbilical cord tissue is 100% versus 63% from umbilical cord blood.
Dental Pulp derived Stem Cells
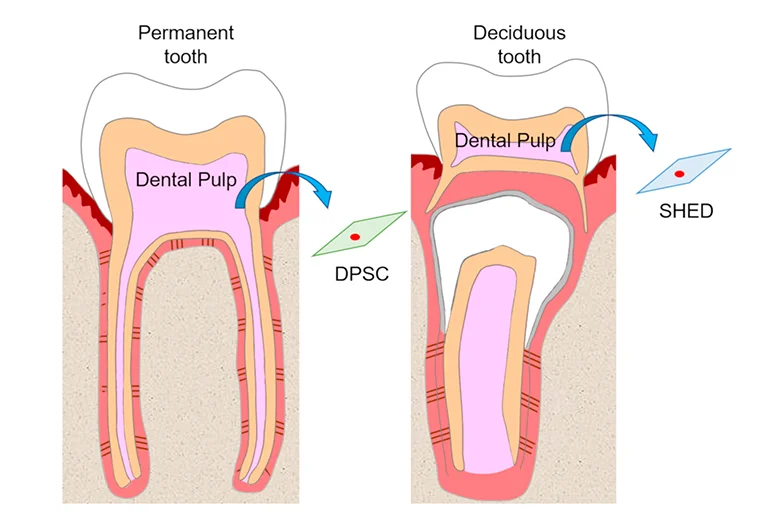
Dental pulp stem cells (DPSCs) can be found within the ‘‘cell rich zone’’ of dental pulp. Their embryonic origin, from neural crests, explains their multipotency and thus may have neurogenic potential that relates to the generation of neurons and their connections, that is, axon guidance. The stem cells found in dental pulp are mesenchymal stem cells. Dental pulp stem cells are excellent for regenerative medicine and tissue engineering applications
Tissue from the dental pulp is a source of neurotrophic factors capable of promoting neuronal survival and neurite outgrowth in vitro and in vivo. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glialderived neurotrophic factor (GDNF), and CXCL12, also known as stromal cell-derived factor-1 (SDF-1), are expressed by dental pulp cells. These factors have also been implicated in axon guidance of the trigeminal ganglion (TG), which subserves sensation to the face and motor control to the masseter muscles of the jaw.
Research & Development is still in progress on Dental Pulp derived Stem Cells.
The efficiency of processing and isolation of mesenchymal stem cells from umbilical cord tissue is 100% versus 63% from umbilical cord blood.
